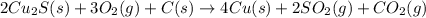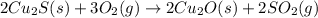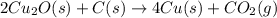There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: (s)(g)(s)(g) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: (s)(s)(s)(g) Write the net chemical equation for the production of copper from copper(I) sulfide, oxygen and carbon. Be sure your equation is balanced.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first st...
Questions

Social Studies, 11.01.2021 16:50

Mathematics, 11.01.2021 16:50





Mathematics, 11.01.2021 16:50


Chemistry, 11.01.2021 16:50

English, 11.01.2021 16:50


Mathematics, 11.01.2021 16:50

Chemistry, 11.01.2021 16:50

Chemistry, 11.01.2021 16:50



Mathematics, 11.01.2021 16:50

Mathematics, 11.01.2021 16:50

Social Studies, 11.01.2021 16:50

History, 11.01.2021 16:50