
Chemistry, 07.03.2020 03:49 queenkimm26
Hydrogen iodide decomposes slowly to H2 and I2 at 600 K. The reaction is second order in HI, and the rate constant is 9.7×10−6M−1s−1. If the initial concentration of HI is 0.110 M. What is its molarity after a reaction time of 5.00 days? Express your answer in moles per liter to two significant figures.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

You know the right answer?
Hydrogen iodide decomposes slowly to H2 and I2 at 600 K. The reaction is second order in HI, and the...
Questions

Mathematics, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00


Biology, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00


Mathematics, 20.11.2021 14:00


Mathematics, 20.11.2021 14:00




Social Studies, 20.11.2021 14:00





Health, 20.11.2021 14:00


Social Studies, 20.11.2021 14:00

![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0537/4912/5ea71.png)
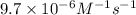
![[A]_o](/tpl/images/0537/4912/9caf5.png) = Initial concentration = 0.110 M
= Initial concentration = 0.110 M![9.7\times 10^{-6}=\frac{1}{5.00}\left (\frac{1}{[A]}-\frac{1}{(0.110)}\right)](/tpl/images/0537/4912/07474.png)
![[A]=0.109M](/tpl/images/0537/4912/e5264.png)


