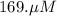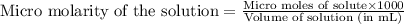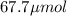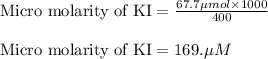Chemistry, 07.03.2020 03:40 ayoismeisalex
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium iodide into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmo1L of the chemist's potassium iodide solution. Round your answer to 3 significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium io...
Questions

History, 04.08.2019 12:30


History, 04.08.2019 12:30

Mathematics, 04.08.2019 12:30


English, 04.08.2019 12:30

Biology, 04.08.2019 12:40





Biology, 04.08.2019 12:40



History, 04.08.2019 12:40


Mathematics, 04.08.2019 12:40

Geography, 04.08.2019 12:40

History, 04.08.2019 12:40