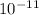Chemistry, 07.03.2020 04:02 hannahpalacios101
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may not be at equilibrium. Identify which mixtures are at equilibrium, if not, indicate which direction the reaction will go to obtain equilibrium (don’t guess, no credit given without supporting evidence). a) [CO] = 1.0*10-3, [Cl2] = 1.0*10-3, [COCl2]= 2.19*10-1 b) [CO] = 3.31*10-6, [Cl2] = 3.31*10-6, [COCl2]= 5.00*10-2 c) [CO] = 4.5*10-7, [Cl2] = 5.73*10-6, [COCl2]= 8.57*10-2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
. Kc = 2.19*10-10 @ 100 oC for the rxn: COCl2(g) CO(g) + Cl2(g) the following mixtures may or may...
Questions

English, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00





Physics, 11.06.2021 14:00

Geography, 11.06.2021 14:00


Physics, 11.06.2021 14:00


Chemistry, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

Mathematics, 11.06.2021 14:00

Spanish, 11.06.2021 14:00



Mathematics, 11.06.2021 14:00

English, 11.06.2021 14:00

![\frac{[CO][Cl2]}{[COCl2]}](/tpl/images/0537/5342/fdabd.png)
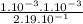 = 0.456.
= 0.456.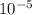
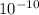 ), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.
), it can be deducted that it is not in equilibrium, since Kc1≠Kc and Kc1 is larger than Kc. When that occurs, it means the reaction is favoring the products, producing more of it. So, the equilibrium is going to the right.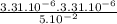 = 0.662.
= 0.662.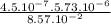 = 3.01.
= 3.01.