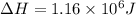Chemistry, 07.03.2020 05:14 spdesch2558
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 182°C. Assume that the specific heat of water is 4.184 J/g · °C for liquid and that the specific heat of steam is 1.99 J/g · °C.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Calculate the amount of energy (in kilojoules) needed to heat 346 g of liquid water from –10 °C to 1...
Questions


Biology, 23.09.2019 16:10



Mathematics, 23.09.2019 16:10

Mathematics, 23.09.2019 16:10




Biology, 23.09.2019 16:10

History, 23.09.2019 16:10



Social Studies, 23.09.2019 16:10





History, 23.09.2019 16:10

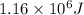
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0537/8966/4a4bb.png)
 = heat required for the reaction
= heat required for the reaction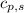 = specific heat of solid water or ice =
= specific heat of solid water or ice = 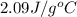
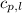 = specific heat of liquid water =
= specific heat of liquid water = 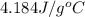
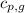 = specific heat of gaseous water =
= specific heat of gaseous water = 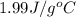
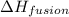 = enthalpy change for fusion =
= enthalpy change for fusion = 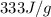
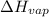 = enthalpy change for vaporization =
= enthalpy change for vaporization = 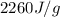
![\Delta H=[346g\times 2.09J/g^oC\times (0-(-80))^oC]+346g\times 333J/g+[346g\times 4.184J/g^oC\times (100-0)^oC]+346g\times 2260J/g+[346g\times 1.99J/g^oC\times (180-100)^oC]](/tpl/images/0537/8966/7d24b.png)