
Chemistry, 07.03.2020 04:45 adrianawalker16
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammonium perchlorate. The following balanced equation represents the reaction.. 3Al + 3NH4ClO4 → Al2O3 + AlCl3 + 3NO + 6H2O How many moles of water are produced from 373 mol Al?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
The fuel used to power the booster rockets on space shuttles is a mixture of aluminum metal and ammo...
Questions


Mathematics, 04.02.2020 10:53


Health, 04.02.2020 10:53

Social Studies, 04.02.2020 10:53

Mathematics, 04.02.2020 10:53

Physics, 04.02.2020 10:54


History, 04.02.2020 10:54



Health, 04.02.2020 10:54

History, 04.02.2020 10:54

History, 04.02.2020 10:54

Social Studies, 04.02.2020 10:54

Mathematics, 04.02.2020 10:54

Biology, 04.02.2020 10:54



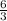 mole of water.
mole of water.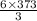 mole of water.
mole of water.

