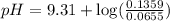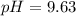Chemistry, 07.03.2020 04:54 andrew6494
A buffer solution contains 0.496 M hydrocyanic acid and 0.399 M sodium cyanide . If 0.0461 moles of sodium hydroxide are added to 225 mL of this buffer, what is the pH of the resulting solution ? (Assume that the volume does not change upon adding sodium hydroxide. )

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
A buffer solution contains 0.496 M hydrocyanic acid and 0.399 M sodium cyanide . If 0.0461 moles of...
Questions

Health, 29.05.2021 18:10

Mathematics, 29.05.2021 18:10

Mathematics, 29.05.2021 18:10

English, 29.05.2021 18:10




Health, 29.05.2021 18:10

Mathematics, 29.05.2021 18:10

Mathematics, 29.05.2021 18:10


Engineering, 29.05.2021 18:10




Mathematics, 29.05.2021 18:10

Chemistry, 29.05.2021 18:10



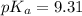
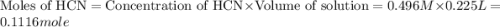
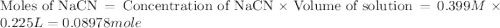
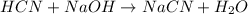
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0537/7961/e961a.png)