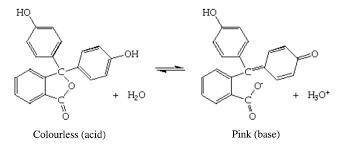
A student carried out an acid-base titration by adding NaOH solution from a buret to an Erlenmeyer flask containing HCl solution and using phenolphthalein as indicator. At the equivalence point, she observed a faint reddish-pink color. However, after a few minutes, the solution gradually turned colorless. What do you suppose happened?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
A student carried out an acid-base titration by adding NaOH solution from a buret to an Erlenmeyer f...
Questions


Chemistry, 27.03.2020 19:50





Mathematics, 27.03.2020 19:50

Chemistry, 27.03.2020 19:50

Physics, 27.03.2020 19:50




Mathematics, 27.03.2020 19:50

Spanish, 27.03.2020 19:50

Mathematics, 27.03.2020 19:50


History, 27.03.2020 19:50

Mathematics, 27.03.2020 19:50


Mathematics, 27.03.2020 19:50