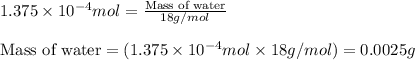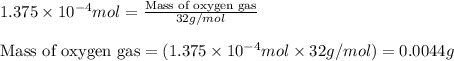Chemistry, 07.03.2020 04:28 pinapunapula
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalanced equation for this reaction iCH4(g)+O2(g)→CO2(g)+H2O(g)This type of reaction is referred to as a complete combustion reaction. A)What mass of carbon dioxide is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. B)What mass of water is produced from the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units. C)What mass of oxygen is needed for the complete combustion of 1.10×10−3 g of methane?Express your answer with the appropriate units.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
When methane (CH4) burns, it reacts with oxygen gas to produce carbon dioxide and water. The unbalan...
Questions

Biology, 08.04.2020 21:00

Biology, 08.04.2020 21:00


Mathematics, 08.04.2020 21:00

English, 08.04.2020 21:00


History, 08.04.2020 21:00





History, 08.04.2020 21:01




Computers and Technology, 08.04.2020 21:01

Biology, 08.04.2020 21:01



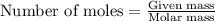 .....(1)
.....(1)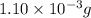
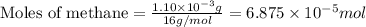
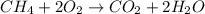
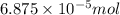 of methane will produce =
of methane will produce = 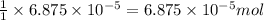 of carbon dioxide
of carbon dioxide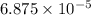 moles
moles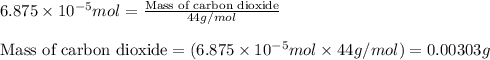
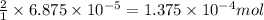 of water
of water moles
moles