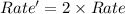Chemistry, 07.03.2020 04:28 blaze9889t
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the effect of doubling the concentration of NaN3 on the rate of the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1



Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the eff...
Questions






Social Studies, 22.07.2020 18:01




Mathematics, 22.07.2020 18:01






Mathematics, 22.07.2020 18:01

English, 22.07.2020 18:01



English, 22.07.2020 18:01

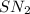 mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:
mechanism, both the reactants take part in the reaction and the rate law for the reaction will be:![Rate=k[NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/f5055.png)
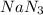 is doubled ,
is doubled , ![Rate'=k[2NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/8f46b.png)
![Rate'=2\times k[NaN_3]^1[CH_3I]^1](/tpl/images/0537/6635/f11c8.png)