Chemistry, 07.03.2020 04:38 isiahemerson0
Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and sodium sulfite (aq) are combined. Note: Sulfites follow the same solubility trends as sulfates.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and sodium sulf...
Questions

Mathematics, 16.09.2019 14:20

Mathematics, 16.09.2019 14:20

English, 16.09.2019 14:20



Physics, 16.09.2019 14:20


Business, 16.09.2019 14:20

Business, 16.09.2019 14:20

Mathematics, 16.09.2019 14:20

Mathematics, 16.09.2019 14:20

English, 16.09.2019 14:20

Health, 16.09.2019 14:20


Mathematics, 16.09.2019 14:20



Social Studies, 16.09.2019 14:20


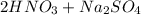 ⇒
⇒ 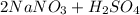
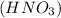
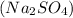
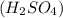
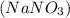
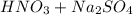 ⇒
⇒