Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3


Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
The standard state of phosphorus at 25∘C25∘C is P4P4. This molecule has four equivalent PP atoms, no...
Questions

Mathematics, 20.11.2020 17:50

History, 20.11.2020 17:50




Mathematics, 20.11.2020 17:50


Mathematics, 20.11.2020 17:50








Mathematics, 20.11.2020 17:50


Physics, 20.11.2020 17:50


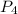 is shown below.
is shown below.