
Chemistry, 07.03.2020 05:06 cnfndbxbfbdb2031
You have an evacuated container of fixed volume and known mass and introduce a known mass of a gas sample. measuring the pressure at constant temperature over time, you are surprised to see it slowly dropping. you measure the mass of the gas-filled container and find that the mass is what it should be - gas plus container - and the mass does not change over time, so you do not have a leak. part a suggest an explanation for your observations. suggest an explanation for your observations.
1. the gas undergoes a chemical reaction that has fewer gas particles in products than in reactants. pressure is directly proportional to number of particles, so the pressure decreased.
2. when the gas was plased into container it obtained some extra kinetic energy after some time this energy was expend, so the speed of the particles reduced and the pressure dropped the gas
3. undergoes a chemical reaction that has more gas particles in products than in reactants.
4. pressure is directly proportional to number of mass.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
You have an evacuated container of fixed volume and known mass and introduce a known mass of a gas s...
Questions


Mathematics, 29.07.2019 11:00


Mathematics, 29.07.2019 11:00


English, 29.07.2019 11:00




Health, 29.07.2019 11:00




English, 29.07.2019 11:00



Mathematics, 29.07.2019 11:00

English, 29.07.2019 11:00

English, 29.07.2019 11:00

Social Studies, 29.07.2019 11:00

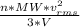
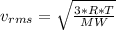 as such for pressure depends only on the number of moles so option 2 is cancelled out
as such for pressure depends only on the number of moles so option 2 is cancelled out


