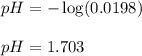Chemistry, 07.03.2020 05:25 suttonfae336
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to remove the top layer of dead skin from the face and ultimately improve the complexion. The value of Ka for monochloroacetic acid is 1.35 ✕ 10−3. Calculate the pH of a 0.31 M solution of monochloroacetic acid.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Monochloroacetic acid (HC2H2ClO2) is a skin irritant that is used in "chemical peels" intended to re...
Questions

Mathematics, 22.01.2021 22:20

English, 22.01.2021 22:20


Biology, 22.01.2021 22:20

Geography, 22.01.2021 22:20

English, 22.01.2021 22:20

Mathematics, 22.01.2021 22:20

Mathematics, 22.01.2021 22:20



English, 22.01.2021 22:20







Mathematics, 22.01.2021 22:20

Computers and Technology, 22.01.2021 22:20

Social Studies, 22.01.2021 22:20

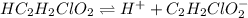
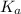 for above equation follows:
for above equation follows:![K_a=\frac{[H^+][C_2H_2ClO_2^-}}{[HC_2H_2ClO_2]}](/tpl/images/0537/9344/a55de.png)
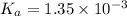
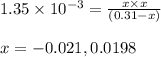
![pH=-\log[H^+]](/tpl/images/0537/9344/cf945.png)