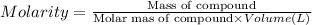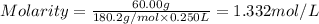Chemistry, 07.03.2020 04:59 forschoolok123456789
A student prepares a solution by dissolving 60.00 g of glucose (molar mass 180.2 g mol-1) in enough distilled water to make 250.0 mL of solution. The molarity of the solution should be reported as
a. 12.01 M
b. 12.0 M
c. 1.332 M
d. 1.33 M
e. 1.3 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
A student prepares a solution by dissolving 60.00 g of glucose (molar mass 180.2 g mol-1) in enough...
Questions


Chemistry, 19.11.2020 21:20

Mathematics, 19.11.2020 21:20

History, 19.11.2020 21:20


Mathematics, 19.11.2020 21:20

History, 19.11.2020 21:20


Mathematics, 19.11.2020 21:20

Mathematics, 19.11.2020 21:20


Mathematics, 19.11.2020 21:20

English, 19.11.2020 21:20

Mathematics, 19.11.2020 21:20


Biology, 19.11.2020 21:20


Mathematics, 19.11.2020 21:20

Arts, 19.11.2020 21:20