
Ammonium perchlorate is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen gas, chlorine gas, oxygen gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

You know the right answer?
Ammonium perchlorate is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decompo...
Questions

Mathematics, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06



Mathematics, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06

Chemistry, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06

Biology, 05.05.2020 11:06


Biology, 05.05.2020 11:06




Mathematics, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06



Mathematics, 05.05.2020 11:06

Mathematics, 05.05.2020 11:06

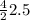 moles of H₂O or 5 moles of H₂O.
moles of H₂O or 5 moles of H₂O.

