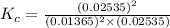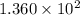Chemistry, 07.03.2020 05:42 jasminebrown72
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M . What is the equilibrium constant if the concentration of A at equilibrium is 0.01365 M ? 2A(aq)+B(aq)⇌2C(aq) Round your answer to one decimal place.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Before any reaction occurs, the concentrations of A and B in the reaction below are each 0.03900 M ....
Questions


Physics, 10.10.2021 16:30



Mathematics, 10.10.2021 16:30


Computers and Technology, 10.10.2021 16:30

Mathematics, 10.10.2021 16:30





Computers and Technology, 10.10.2021 16:30

Business, 10.10.2021 16:30

SAT, 10.10.2021 16:30


Chemistry, 10.10.2021 16:30

Mathematics, 10.10.2021 16:30


Computers and Technology, 10.10.2021 16:30

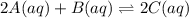
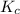 of the given reaction is as follows.
of the given reaction is as follows.![K_{c} = \frac{[C]^{2}}{[A]^{2}[B]}](/tpl/images/0538/0202/8ca5b.png)