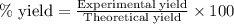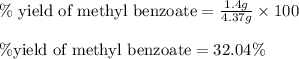Chemistry, 07.03.2020 05:25 livvyr0cks
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presence of sulfuric acid as a catalyst. A reaction was performed in which 3.6 g of benzoic acid was reacted with excess methanol to make 1.4 g of methyl benzoate. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1


Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Consider the Fischer ester synthesis of methyl benzoate from benzoic acid and methanol in the presen...
Questions


Social Studies, 08.01.2021 16:40



Mathematics, 08.01.2021 16:40

Chemistry, 08.01.2021 16:40

Mathematics, 08.01.2021 16:40


Chemistry, 08.01.2021 16:40



Chemistry, 08.01.2021 16:40


Social Studies, 08.01.2021 16:40


Mathematics, 08.01.2021 16:40


English, 08.01.2021 16:40

Mathematics, 08.01.2021 16:40

Mathematics, 08.01.2021 16:40

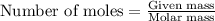 .....(1)
.....(1)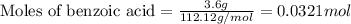
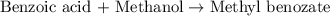
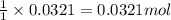 of methyl benzoate
of methyl benzoate