Chemistry, 07.03.2020 05:26 kfcnkfnmnfk9513
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to the equation2NO2(g)?2NO(g)+O2(g)At equilibrium the density of the gas mixture is 0.525g/L at 0.745atm . Calculate Kc for the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1


You know the right answer?
A sample of pure NO2 is heated to 337?C at which temperature it partially dissociates according to t...
Questions

Mathematics, 21.08.2019 04:00



Mathematics, 21.08.2019 04:00

History, 21.08.2019 04:00


Biology, 21.08.2019 04:00

Biology, 21.08.2019 04:00

History, 21.08.2019 04:00

Mathematics, 21.08.2019 04:00

Social Studies, 21.08.2019 04:00


History, 21.08.2019 04:00

English, 21.08.2019 04:00

Physics, 21.08.2019 04:00

Mathematics, 21.08.2019 04:00

Chemistry, 21.08.2019 04:00


Mathematics, 21.08.2019 04:00

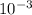
![\frac{[NO]^{2} .[O2]}{[NO2]^{2} }](/tpl/images/0537/9415/78cff.png)
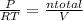
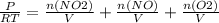
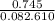 = 0.015 mol/L
= 0.015 mol/L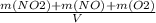
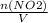 +M(NO)·
+M(NO)·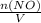 +M(O2)·
+M(O2)·