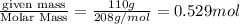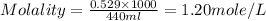Chemistry, 07.03.2020 05:30 bobduncan1086
A chemist prepares a solution of barium chloride by measuring out of barium chloride into a volumetric flask and filling the flask to the mark with water. Calculate the concentration in of the chemist's barium chloride solution. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
A chemist prepares a solution of barium chloride by measuring out of barium chloride into a volumetr...
Questions


Mathematics, 20.11.2020 06:20


Mathematics, 20.11.2020 06:20

Mathematics, 20.11.2020 06:20



English, 20.11.2020 06:20

Computers and Technology, 20.11.2020 06:20

Mathematics, 20.11.2020 06:20

History, 20.11.2020 06:20

Mathematics, 20.11.2020 06:20

Mathematics, 20.11.2020 06:20

English, 20.11.2020 06:20


Mathematics, 20.11.2020 06:20


Mathematics, 20.11.2020 06:20

Mathematics, 20.11.2020 06:20

Social Studies, 20.11.2020 06:20

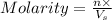
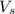 = volume of solution in L
= volume of solution in L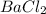 (solute) =
(solute) =