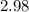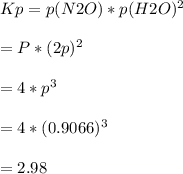Chemistry, 07.03.2020 06:17 missheather0309
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation:
NH4NO3(s) N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.72 bar at a temperature of 500.°C. Calculate Kp.
a.
1.64
b.
0.822
c.
2.98
d.
80.5
e.
0.745

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed...
Questions

Computers and Technology, 26.02.2021 06:40

Biology, 26.02.2021 06:40


Chemistry, 26.02.2021 06:40



English, 26.02.2021 06:40



History, 26.02.2021 06:40

Mathematics, 26.02.2021 06:40

History, 26.02.2021 06:40


Mathematics, 26.02.2021 06:40

Mathematics, 26.02.2021 06:40

History, 26.02.2021 06:40


Mathematics, 26.02.2021 06:40


Mathematics, 26.02.2021 06:40