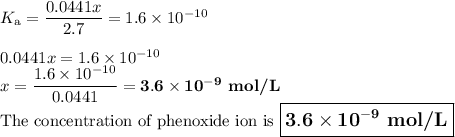Chemistry, 07.03.2020 06:00 andrewmena05
Calculate the pH of a solution that contains 2.7 M HF and 2.7 M HOC6H5. Also, calculate the concentration of OC6H5- in this solution at equilibrium. Ka(HF) = 7.2×10-4; Ka(HOC6H5) = 1.6×10-10. pH = [OC6H5-] = M

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
Calculate the pH of a solution that contains 2.7 M HF and 2.7 M HOC6H5. Also, calculate the concentr...
Questions


English, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01








Mathematics, 20.10.2020 22:01




Biology, 20.10.2020 22:01

Computers and Technology, 20.10.2020 22:01

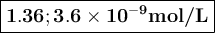
![K_{\text{a}} = \dfrac{\text{[H}_{3}\text{O}^{+}] \text{F}^{-}]} {\text{[HF]}} = 7.2 \times 10^{-4}\\\\\dfrac{x^{2}}{2.7 - x} = 7.2 \times 10^{-4}\\\\\text{Check for negligibility of }x\\\\\dfrac{2.7}{7.2 \times 10^{-4}} = 4000 400\\\\\therefore x \ll 2.7\\\dfrac{x^{2}}{2.7} = 7.2 \times 10^{-4}\\\\x^{2} = 2.7 \times 7.2 \times 10^{-4} = 1.94 \times 10^{-3}\\x = 0.0441\\\text{[H$_{3}$O$^{+}$]}= \text{x mol$\cdot$L$^{-1}$} = \text{0.0441 mol$\cdot$L$^{-1}$}](/tpl/images/0538/0586/c13f6.png)
![\text{pH} = -\log{\rm[H_{3}O^{+}]} = -\log{0.0441} = \large \boxed{\mathbf{1.36}}](/tpl/images/0538/0586/4731b.png)