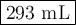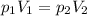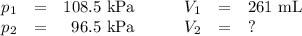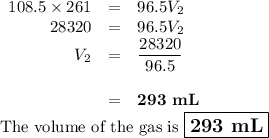Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
Neon gas has a volume of 261 ml and a pressure of 108.5 kPa. What volume will the gas occupy at 96.5...
Questions

English, 14.11.2020 16:10



Mathematics, 14.11.2020 16:10




Mathematics, 14.11.2020 16:10


History, 14.11.2020 16:10

Biology, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10

Computers and Technology, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10

Chemistry, 14.11.2020 16:10

Chemistry, 14.11.2020 16:10


History, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10