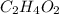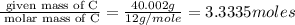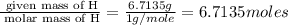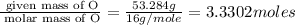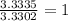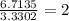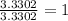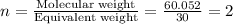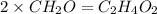Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
The percent composition by mass of an unknown compound with a molecular mass of 60.052 amu is 40.002...
Questions


Geography, 13.04.2021 18:30

English, 13.04.2021 18:30

History, 13.04.2021 18:30




Mathematics, 13.04.2021 18:30

History, 13.04.2021 18:30

Computers and Technology, 13.04.2021 18:30

Spanish, 13.04.2021 18:30

Mathematics, 13.04.2021 18:30




Mathematics, 13.04.2021 18:30



Mathematics, 13.04.2021 18:30

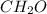 and
and