
Chemistry, 09.03.2020 16:59 BigDough9090
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solution. Calculate the pOH of the resulting solution.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2
You know the right answer?
375 mL of a 0.88 M potassium hydroxide solution is added to 496 mL of a 0.76 M cesium hydroxide solu...
Questions


Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00


English, 19.04.2021 14:00

Chemistry, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00


Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Physics, 19.04.2021 14:00

Advanced Placement (AP), 19.04.2021 14:00

English, 19.04.2021 14:00



History, 19.04.2021 14:00

History, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

Mathematics, 19.04.2021 14:00

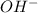 in 375 mL of 0.88 M of KOH =
in 375 mL of 0.88 M of KOH = 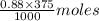 = 0.33 moles
= 0.33 moles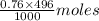 = 0.38 moles
= 0.38 moles![[OH^{-}]](/tpl/images/0538/8908/e46dd.png) =
= 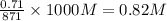
![pOH=-log[OH^{-}]=-log(0.82)=0.086](/tpl/images/0538/8908/1f1db.png)



