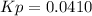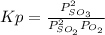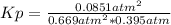Chemistry, 09.03.2020 23:46 TerronRice
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K where they react to form SO3 (g). If the vessel contained 0.669 atm SO2 (g), 0.395 atm O2 (g) and 0.0851 atom SO3 (g) after the system has reached equilibrium, what is the equilibrium constant, Kp, for the reaction

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K w...
Questions




Mathematics, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

Business, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01

Computers and Technology, 20.09.2020 14:01









History, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01