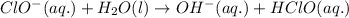Chemistry, 10.03.2020 00:13 bercishicicorbin
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. Write the net acid-base reaction that occurs when dissolved NaClO reacts with water. (Use the lowest possible coefficients. Omit states-of-matter in your answer.)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water...
Questions



Business, 15.12.2020 18:00



Computers and Technology, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00

English, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00




Mathematics, 15.12.2020 18:00

Mathematics, 15.12.2020 18:00

English, 15.12.2020 18:00

Computers and Technology, 15.12.2020 18:00


Mathematics, 15.12.2020 18:00

Chemistry, 15.12.2020 18:00

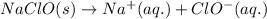
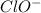 reacts with water to form conjugate acid.
reacts with water to form conjugate acid.