

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
An equilibrium mixture of CO, O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0025 M...
Questions




Mathematics, 19.05.2020 15:02

Mathematics, 19.05.2020 15:02

Physics, 19.05.2020 15:02




History, 19.05.2020 15:02

Biology, 19.05.2020 15:02

English, 19.05.2020 15:02


English, 19.05.2020 15:02


Mathematics, 19.05.2020 15:02

History, 19.05.2020 15:02

Mathematics, 19.05.2020 15:02

English, 19.05.2020 15:02

Mathematics, 19.05.2020 15:02

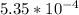 M
M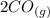 +
+ 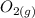 ⇄
⇄ 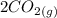
![K_c =\frac{[CO_2]^2}{[CO]^2[O_2]}](/tpl/images/0539/1399/a6d01.png)
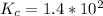 ; Then:
; Then: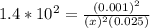
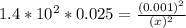
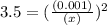
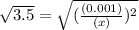
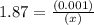
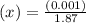
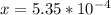 M
M

