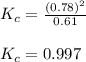Chemistry, 09.03.2020 23:59 pollywallythecat
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium described by the equation N2O4(g) 2NO2(g) If at equilibrium the N2O4 is 39% dissociated, what is the value of the equilibrium constant, Kc, for the reaction under these conditions

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium descr...
Questions

Computers and Technology, 15.10.2020 04:01

Mathematics, 15.10.2020 04:01

Business, 15.10.2020 04:01


Geography, 15.10.2020 04:01


Law, 15.10.2020 04:01

History, 15.10.2020 04:01


Mathematics, 15.10.2020 04:01

Mathematics, 15.10.2020 04:01



Mathematics, 15.10.2020 04:01

English, 15.10.2020 04:01

English, 15.10.2020 04:01

English, 15.10.2020 04:01

Social Studies, 15.10.2020 04:01


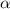 = 0.39
= 0.39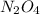 , c =
, c = 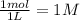
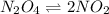
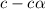
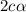
![N_2O_4=c-c\alpha =[1-(1\times 0.39)]=0.61M](/tpl/images/0539/1468/f8a46.png)
![NO_2=2c\alpha =[2\times 1\times 0.39]=0.78M](/tpl/images/0539/1468/5fcc4.png)
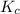 for above equation follows:
for above equation follows:![K_{c}=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0539/1468/1a559.png)