
Chemistry, 10.03.2020 00:00 CarQuestionl6367
Assume that you dissolve 10.1 g of a mixture of NaOH and Ba(OH)2 in 253.0 mL of water and titrate with 1.53 M hydrochloric acid. The titration is complete after 107.8 mL of the acid has been added. What is the mass (in grams) of NaOH in the mixture?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2


Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Assume that you dissolve 10.1 g of a mixture of NaOH and Ba(OH)2 in 253.0 mL of water and titrate wi...
Questions

English, 30.06.2019 19:10

Spanish, 30.06.2019 19:10

Mathematics, 30.06.2019 19:10

English, 30.06.2019 19:10

Chemistry, 30.06.2019 19:10






Mathematics, 30.06.2019 19:10

Computers and Technology, 30.06.2019 19:10



History, 30.06.2019 19:10



Mathematics, 30.06.2019 19:10


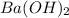 be y.
be y.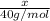
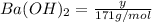
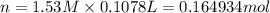
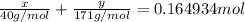
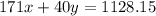 ..[2]
..[2]

