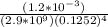Chemistry, 10.03.2020 00:22 toshahoskins0098
A solution is made that is 1.2×10−3 M in Zn(NO3)2 and 0.130 M in NH3. After the solution reaches equilibrium, what concentration of Zn2+(aq) remains?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
A solution is made that is 1.2×10−3 M in Zn(NO3)2 and 0.130 M in NH3. After the solution reaches equ...
Questions



Mathematics, 28.09.2019 22:30





History, 28.09.2019 22:30


English, 28.09.2019 22:30

Health, 28.09.2019 22:30



Social Studies, 28.09.2019 22:30






History, 28.09.2019 22:30

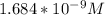
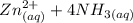 ⇄
⇄ ![[Zn(NH_3)_4^+_{(aq)}]](/tpl/images/0539/2581/6fbcb.png)
![[Zn^{2+}]](/tpl/images/0539/2581/9c01a.png) at equilibrium.
at equilibrium.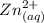

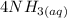 ⇄
⇄ 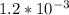
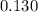
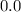

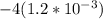

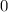
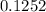
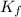

![\frac{[Zn(NH_3)_4^{2+}]}{[Zn^{2+}][NH_3]^4}](/tpl/images/0539/2581/585d3.png) ⇒
⇒ ![\frac{[Zn(NH_3)_4^{2+}]}{[K_fNH_3]^4}](/tpl/images/0539/2581/6256e.png)