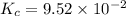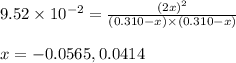He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) + CCl4(g)→ 2CH2Cl2(g)
Calculate the equilibrium concentrations of reactants and product when 0.310 moles of CH4 and 0.310 moles of CCl4 are introduced into a 1.00 L vessel at 350 K.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
He equilibrium constant, Kc, for the following reaction is 9.52×10-2 at 350 K:
CH4(g) +...
CH4(g) +...
Questions

English, 08.10.2020 01:01


History, 08.10.2020 01:01

Mathematics, 08.10.2020 01:01



Chemistry, 08.10.2020 01:01

Computers and Technology, 08.10.2020 01:01






Geography, 08.10.2020 01:01




Engineering, 08.10.2020 01:01

English, 08.10.2020 01:01

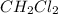 are 0.2686 M, 0.2686 M and 0.0828 M respectively.
are 0.2686 M, 0.2686 M and 0.0828 M respectively.
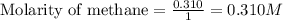
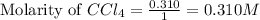
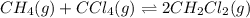
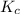 for above equation follows:
for above equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0539/2756/bf52a.png)