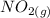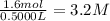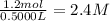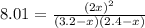Chemistry, 10.03.2020 00:27 TabbyKun00
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becomes possible: +NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.01 at the temperature of the flask. Calculate the equilibrium molarity of NO3 . Round your answer to two decimal places.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

You know the right answer?
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becom...
Questions


Physics, 15.01.2021 05:00


English, 15.01.2021 05:00





Mathematics, 15.01.2021 05:00



Chemistry, 15.01.2021 05:00

Arts, 15.01.2021 05:00

Chemistry, 15.01.2021 05:00

Mathematics, 15.01.2021 05:00

Mathematics, 15.01.2021 05:00

Arts, 15.01.2021 05:00


Physics, 15.01.2021 05:00

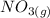 is 1.60 M
is 1.60 M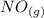 ⇄2
⇄2