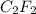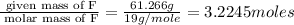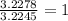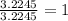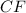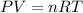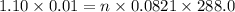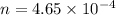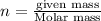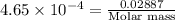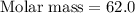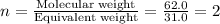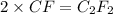Chemistry, 10.03.2020 00:29 jjimenez0276
A 0.02887 g sample of gas occupies 10.0 mL at 288.0 K and 1.10 atm. Upon further analysis, the compound is found to be 38.734 % C and 61.266 % F . What is the molecular formula of the compound?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3


Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
A 0.02887 g sample of gas occupies 10.0 mL at 288.0 K and 1.10 atm. Upon further analysis, the compo...
Questions

Mathematics, 07.05.2020 05:09





Mathematics, 07.05.2020 05:09



Arts, 07.05.2020 05:09


Computers and Technology, 07.05.2020 05:09

History, 07.05.2020 05:09

English, 07.05.2020 05:09




Mathematics, 07.05.2020 05:09