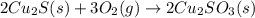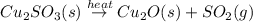Chemistry, 10.03.2020 00:40 leysirivera23ovez6n
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first. subsequently, upon heating, the copper sulfite thermally decomposes to copper oxide and sulfur dioxide. Write balanced chemical equations for these two reactions

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

Chemistry, 23.06.2019 16:00
Henry moseley used x-ray experiments to determine the atomic number of elements. how did his discovery contribute to the development of the periodic table? a.it confirmed that elements should be arranged in strict order of increasing atomic mass. b.it led to elements with similar atomic numbers being grouped together. c.it allowed the elements to be placed in strict order of increasing atomic number. d.it showed that the way mendeleev grouped elements together was completely wrong.
Answers: 1
You know the right answer?
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first....
Questions

Mathematics, 22.10.2020 19:01



History, 22.10.2020 19:01


Social Studies, 22.10.2020 19:01


History, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01

Computers and Technology, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01



Spanish, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01


Social Studies, 22.10.2020 19:01