
Chemistry, 10.03.2020 01:24 jolleyrancher78
Calculate the pH at the equivalence point for the titration of 0.200 M 0.200 M methylamine ( CH 3 NH 2 ) (CH3NH2) with 0.200 M HCl . 0.200 M HCl. The K b Kb of methylamine is 5.0 × 10 − 4 . 5.0×10−4. pH =

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3
You know the right answer?
Calculate the pH at the equivalence point for the titration of 0.200 M 0.200 M methylamine ( CH 3 NH...
Questions


English, 29.01.2021 18:00



English, 29.01.2021 18:00


English, 29.01.2021 18:00

Mathematics, 29.01.2021 18:00

Mathematics, 29.01.2021 18:00


Mathematics, 29.01.2021 18:00


English, 29.01.2021 18:00






Mathematics, 29.01.2021 18:00

Chemistry, 29.01.2021 18:00

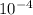 )
)

