
Chemistry, 10.03.2020 01:44 tobyhollingsworth178
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g of water to yield the following reaction: NaOH + HBr → Na+(aq) + Br-(aq) + H2O(l) After mixing the temperature rises to 88.5 oC. Calculate the change in enthalpy of this reaction. Specific heat of the solution = 4.184 J/(g oC) State your answer in kJ with 3 significant figures. Don't forget to enter the unit behind the numerical answer. The molecular weight of NaOH is 40.0 g/mol, and the molecular weight of HBr is 80.9 g/mol. ΔH =

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g...
Questions

Biology, 28.08.2019 17:00


Physics, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00

Mathematics, 28.08.2019 17:00


Biology, 28.08.2019 17:00

Biology, 28.08.2019 17:00


Physics, 28.08.2019 17:00




Mathematics, 28.08.2019 17:00

Arts, 28.08.2019 17:00


Social Studies, 28.08.2019 17:00




 = final temperature =
= final temperature = 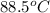
 = initial temperature =
= initial temperature = 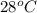
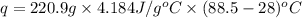
 ( J = 0.001 kJ)
( J = 0.001 kJ)

 = enthalpy change = ?
= enthalpy change = ?


