
Chemistry, 10.03.2020 03:04 ijustneedhelp29
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D (aq) Kc = 178. If initially the concentrations of the chemical species are as follows: [A]o = 0.364 M [B]o = 0.170 M [C]o = 0.132 M [D]o = 0.932 M to which direction will the reaction run to reach equilibrium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D...
Questions


English, 28.08.2019 12:10


Biology, 28.08.2019 12:10



Social Studies, 28.08.2019 12:10


Social Studies, 28.08.2019 12:10


Mathematics, 28.08.2019 12:10



Social Studies, 28.08.2019 12:10



Mathematics, 28.08.2019 12:10

Biology, 28.08.2019 12:10


English, 28.08.2019 12:10

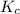 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while 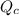 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.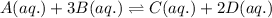
![Q_c=\frac{[D]^2[C]}{[A][B}^3}](/tpl/images/0539/7435/19a81.png)
![[A]_o=0.364M](/tpl/images/0539/7435/6e4ee.png)
![[B]_o=0.170M](/tpl/images/0539/7435/df462.png)
![[C]_o=0.132M](/tpl/images/0539/7435/924d4.png)
![[D]_o=0.932M](/tpl/images/0539/7435/7f66b.png)
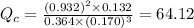

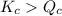 ; the reaction is product favored.When
; the reaction is product favored.When 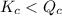 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 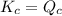 ; the reaction is in equilibrium.
; the reaction is in equilibrium.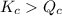 , the reaction will be favoring product side.
, the reaction will be favoring product side.

