

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 11:20
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1
You know the right answer?
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN)2- forms? Ksp...
Questions

English, 30.11.2021 17:50

Computers and Technology, 30.11.2021 17:50


Business, 30.11.2021 17:50



Physics, 30.11.2021 17:50



Biology, 30.11.2021 17:50

Computers and Technology, 30.11.2021 17:50

History, 30.11.2021 17:50

Business, 30.11.2021 17:50




Business, 30.11.2021 17:50



![Ksp = [Ag^{+}][Cl^{-}] = 1.8 \cdot 10^{-10}](/tpl/images/0539/7706/583a7.png) (1)
(1) ![Kf = \frac{[Ag(CN)_{2}^{-}]}{[Ag^{+}][CN^{-}]^{2}} = 1.0 \cdot 10^{21}](/tpl/images/0539/7706/daa6c.png) (2)
(2)![Keq = \frac{[Ag(CN)_{2}^{-})][Cl^{-}]}{[CN^{-}]^{2}}](/tpl/images/0539/7706/f78ba.png) (3)
(3)![Keq = \frac{[Ag(CN)_{2}^{-})]}{[CN^{-}]^{2}} \frac{Ksp}{[Ag^{+}]} = Kf \cdot Ksp = 1.0 \cdot 10^{21} \cdot 1.8 \cdot 10^{-10} = 1.8 \cdot 10^{11}](/tpl/images/0539/7706/79edb.png)
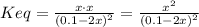 (4)
(4)

