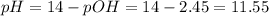Chemistry, 10.03.2020 03:30 morganmattal
Calculate the pH and concentrations of CH 3 NH 2 and CH 3 NH + 3 in a 0.0317 M methylamine ( CH 3 NH 2 ) solution. The K b of CH 3 NH 2 is 4.47 × 10 − 4 .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
Calculate the pH and concentrations of CH 3 NH 2 and CH 3 NH + 3 in a 0.0317 M methylamine ( CH 3 NH...
Questions


Mathematics, 04.12.2020 17:40


Chemistry, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40


Mathematics, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40


Chemistry, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40

English, 04.12.2020 17:40






English, 04.12.2020 17:40

Mathematics, 04.12.2020 17:40

![K_{b} = \frac{[CH_{3}NH_{3}^{+}][OH^{-}]}{[CH_{3}NH_{2}]}](/tpl/images/0539/8001/0cb42.png)
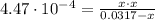
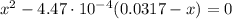 (2)
(2) ![pOH = -log [OH^{-}] = -log (0.00355) = 2.45](/tpl/images/0539/8001/ff2f0.png)