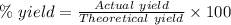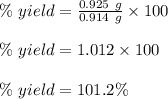Chemistry, 10.03.2020 03:30 ellycleland16
Starting with 0.657 g of lead(II) nitrate, a student collects 0.925 g of precipitate. If the calculated mass of precipitate is 0.914 g, what is the percent yield

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
Starting with 0.657 g of lead(II) nitrate, a student collects 0.925 g of precipitate. If the calcula...
Questions



Mathematics, 19.04.2021 22:00




Spanish, 19.04.2021 22:00



Mathematics, 19.04.2021 22:00

History, 19.04.2021 22:00


English, 19.04.2021 22:00

History, 19.04.2021 22:00

Mathematics, 19.04.2021 22:00

Chemistry, 19.04.2021 22:00


Mathematics, 19.04.2021 22:00

Mathematics, 19.04.2021 22:00