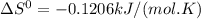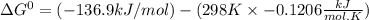Chemistry, 10.03.2020 03:39 puchie1225
The following reaction has the thermodynamic values at 298 K: ΔH° = -136.9 kJ/mol and ΔS° = -120.6 J/mol K. Calculate ΔG° at 298 K for this reaction in kJ/mol (Enter your answer to four significant figures.):

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2


Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

You know the right answer?
The following reaction has the thermodynamic values at 298 K: ΔH° = -136.9 kJ/mol and ΔS° = -120.6 J...
Questions

History, 16.02.2021 21:40


Mathematics, 16.02.2021 21:40


Health, 16.02.2021 21:40


History, 16.02.2021 21:40

Engineering, 16.02.2021 21:40




Mathematics, 16.02.2021 21:40


Mathematics, 16.02.2021 21:40


English, 16.02.2021 21:40



Mathematics, 16.02.2021 21:40

Arts, 16.02.2021 21:40

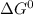 at 298 K is -101.0 kJ/mol
at 298 K is -101.0 kJ/mol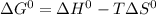
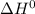 , T and
, T and 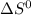 represent change in free energy in standard state, change in enthalpy in standard state, temperature in kelvin scale and change in entropy in standard state.
represent change in free energy in standard state, change in enthalpy in standard state, temperature in kelvin scale and change in entropy in standard state.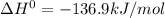 , T = 298 K and
, T = 298 K and