
Chemistry, 10.03.2020 04:11 fluffyunicorn59803
Onsider the following reaction at equilibrium:
C(s)+H2O(g)⇌CO(g)+H2(g)
Predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances.
A. C is added to the reaction mixture.
B. H2Ois condensed and removed from the reaction mixture.
C. CO is added to the reaction mixture.
D. H2 is removed from the reaction mixture.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1
You know the right answer?
Onsider the following reaction at equilibrium:
C(s)+H2O(g)⇌CO(g)+H2(g)
Pre...
C(s)+H2O(g)⇌CO(g)+H2(g)
Pre...
Questions

Chemistry, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30

English, 30.06.2019 20:30

Spanish, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30



Chemistry, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Spanish, 30.06.2019 20:30


Physics, 30.06.2019 20:30

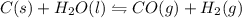
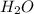 is condensed and removed from the reaction mixture.
is condensed and removed from the reaction mixture.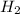 is removed from the reaction mixture.
is removed from the reaction mixture.

