
Chemistry, 10.03.2020 04:50 MathChic68
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.10M and [I2]=2.50M . The equilibrium concentration of I2 is 0.0800 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions



Mathematics, 31.08.2021 16:20

Biology, 31.08.2021 16:20

Mathematics, 31.08.2021 16:20

Mathematics, 31.08.2021 16:20


Mathematics, 31.08.2021 16:20

Biology, 31.08.2021 16:20

History, 31.08.2021 16:20

Biology, 31.08.2021 16:20


Mathematics, 31.08.2021 16:20



Social Studies, 31.08.2021 16:30

History, 31.08.2021 16:30

Biology, 31.08.2021 16:30

Mathematics, 31.08.2021 16:30

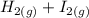 ⇌
⇌ 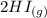
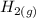

 ⇌
⇌ ![K_c = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0540/0427/6b81e.png)

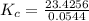
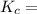 430.62
430.62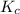 ≅ 431
≅ 431 


