
Chemistry, 10.03.2020 04:52 Katie123amazing
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are constant over the temperature interval at their values at 298.15 K. Molar heat capacities of NO(g), N2(g), and O2(g) at 298.15 K are 29.86, 29.13, and 29.38 J⋅K−1⋅mol−1. The standard enthalpy of formation of NO(g) is 91.3 kJ⋅mol−1 at 298.15 K.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are c...
Questions


Mathematics, 18.11.2020 03:40

History, 18.11.2020 03:40


Mathematics, 18.11.2020 03:40


Mathematics, 18.11.2020 03:40


English, 18.11.2020 03:40

Law, 18.11.2020 03:40

Mathematics, 18.11.2020 03:40

Mathematics, 18.11.2020 03:40


Mathematics, 18.11.2020 03:40



Mathematics, 18.11.2020 03:40

Arts, 18.11.2020 03:40


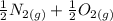 ------>
------>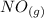
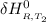 =
= 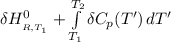
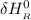 = enthalpy of reaction
= enthalpy of reaction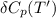 = the difference in the heat capacities of the products and the reactants.
= the difference in the heat capacities of the products and the reactants.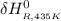 =
= 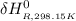

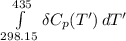
![1(91300 J.mol^{-1} ) +\int\limits^{435}_{298.15} [{(29.86)-\frac{1}{2}(29.38)-\frac{1}{2}29.13}]J.K^{-1}.mol^{-1} \, dT'](/tpl/images/0540/0534/3b971.png)


