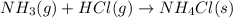Chemistry, 10.03.2020 04:48 Hollywood0122
Using the Lewis concept of acids and bases, identify the Lewis acid and base in each of the following reactions: Fe(NO3)3(s)+6H2O(l)→Fe(H2O)63+(aq)+ 3NO3−(aq) NH3(g)+HCl(g)→NH4Cl(s) Drag the appropriate items to their respective bins.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Using the Lewis concept of acids and bases, identify the Lewis acid and base in each of the followin...
Questions



Mathematics, 31.05.2020 03:01


History, 31.05.2020 03:01




Mathematics, 31.05.2020 03:01


Mathematics, 31.05.2020 03:01

Mathematics, 31.05.2020 03:01


Computers and Technology, 31.05.2020 03:01



Biology, 31.05.2020 03:01



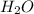 is a Lewis-base and
is a Lewis-base and  is a Lewis-acid.
is a Lewis-acid.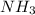 is a Lewis-base and
is a Lewis-base and 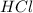 is a Lewis-acid.
is a Lewis-acid.![Fe(NO_3)_3(s)+6H_2O(l)\rightarrow [Fe(H_2O)_6]^{3+}(aq)+3NO_3^-(aq)](/tpl/images/0540/0340/85418.png)