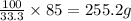Chemistry, 10.03.2020 04:47 morenodonaldo762
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3% w/w solution of acetic acid in ethanol. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3...
Questions

Advanced Placement (AP), 10.11.2019 19:31

Chemistry, 10.11.2019 19:31

History, 10.11.2019 19:31

Mathematics, 10.11.2019 19:31




World Languages, 10.11.2019 19:31


Biology, 10.11.2019 19:31


Social Studies, 10.11.2019 19:31


Physics, 10.11.2019 19:31

Computers and Technology, 10.11.2019 19:31





History, 10.11.2019 19:31