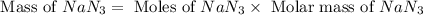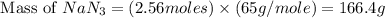The air bags in cars are inflated when a collision triggers the explosive, highly exothermic decomposition of sodium azide (NaN3): 2NaN3(s) → 2Na(s) + 3N2(g) The passenger-side air bag in a typical car must fill a space approximately four times as large as the driver-side bag to be effective. Calculate the mass of sodium azide required to fill a 113-L air bag. Assume the pressure in the car is 1.00 atm and the temperature of N2 produced is 85°C.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2


Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
The air bags in cars are inflated when a collision triggers the explosive, highly exothermic decompo...
Questions


History, 29.01.2020 17:53

English, 29.01.2020 17:53





English, 29.01.2020 17:53

Mathematics, 29.01.2020 17:53


Mathematics, 29.01.2020 17:53

Advanced Placement (AP), 29.01.2020 17:53



History, 29.01.2020 17:54



English, 29.01.2020 17:54


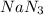 required is, 166.4 grams.
required is, 166.4 grams.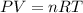
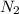 gas = 1.00 atm
gas = 1.00 atm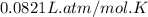
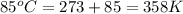
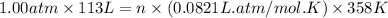
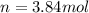
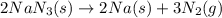
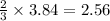 moles of
moles of