
Chemistry, 10.03.2020 05:58 ayoismeisalex
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M Ca(OH)2, and 33.93 mL of the Ca(OH)2 solution is required to reach the equivalence point. What is the molarity of the acetic acid? Group of answer choices

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 06:30
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M...
Questions


Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

History, 07.05.2021 01:00





Arts, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00


Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00


Chemistry, 07.05.2021 01:00


Mathematics, 07.05.2021 01:00

Mathematics, 07.05.2021 01:00

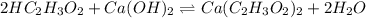
 neutralizes 2 moles of
neutralizes 2 moles of 






