Chemistry, 10.03.2020 06:21 joseroblesrivera123
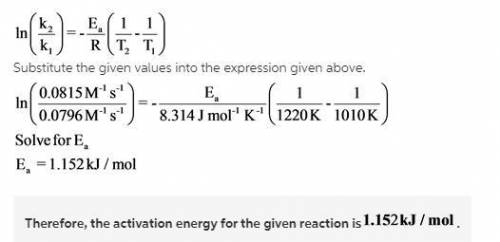
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. The decomposition of nitric oxide (NO)(NO) to N2N2 and O2O2 is second order with a rate constant of 0.0796 M−1⋅s−1M−1⋅s−1 at 737∘C∘C and 0.0815 M−1⋅s−1M−1⋅s−1 at 947∘C∘C.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01


History, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01






Mathematics, 05.10.2020 15:01




Physics, 05.10.2020 15:01